Initially developed as a diagnostic imaging tool to enhance local tumor staging and characterize mucosal and submucosal lesions, endoscopic ultrasound (EUS) has evolved into a valuable therapeutic modality for managing a variety of gastrointestinal and nongastrointestinal disorders.
As imaging quality and available accessories have improved, therapeutic EUS applications have expanded to include extraluminal structures. Established and emerging interventions include, but are not limited to, EUS-guided angiotherapy, tumor ablation, transluminal gallbladder drainage, acute postsurgical fluid collection drainage, and celiac plexus neurolysis for pain management.
We present selected cases from our files that exhibit a few of these relatively novel approaches from the "frontiers" of EUS.
A 63-year-old male patient with alcoholic cirrhosis and pancreatitis presented with his fifth episode of hematemesis requiring hospitalization. Evaluation at other facilities demonstrated blood filling the proximal duodenum, and on one occasion endoscopic examination showed blood emanating from the major papilla. After receiving more than 18 units of packed red blood cells, the patient was transferred to our facility for further evaluation.
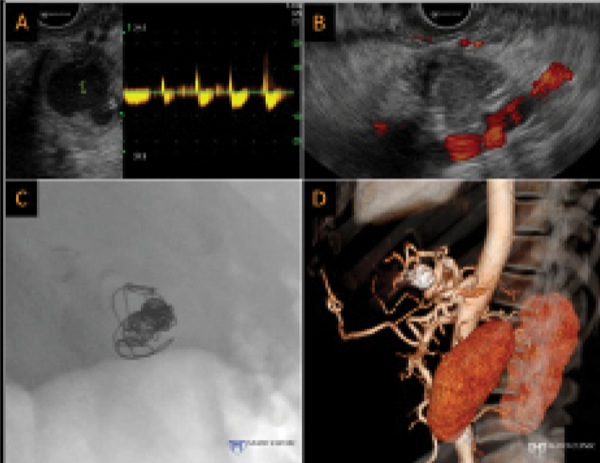
Computed tomographic angiography (CTA) revealed a splenic artery aneurysm resulting in hemosuccus pancreaticus. Interventional radiological procedures were unable to localize the source of bleeding and uncovered no obvious aneurysm. During EUS, pulse Doppler demonstrated flow within the aneurysm (Figure 1A), and we completed EUS-guided coil injection of the aneurysm successfully with fine needle aspiration (FNA) using a 19-gauge needle. After coil insertion, power Doppler demonstrated an absence of flow (Figure 1B). The coils were seen easily on fluoroscopy (Figure 1C), and a follow-up CTA revealed an absence of flow within the aneurysm (Figure 1D).
EUS-guided angiography employs a variety of agents—including fibrin sealants, epinephrine, alcohol, hyaluronate, and microcoils—to manage bleeding originating from a variety of sources: gastric and nongastric varices (esophageal, duodenal, pericholedochal, rectal, and peristomal), esophageal cancer, Dieulafoy lesions, stromal tumors, ulcers (gastric, duodenal, and luminal anastomotic), Brunner's gland hamartoma, luminal wall direct infiltration and metastasis from distant tumors (renal cell carcinoma, colon adenocarcinoma, prostate cancer), pseudoaneurysms (pancreatic, splenic artery, and mesenteric), arteriovenous malformations (duodenal and colon), and unclassified and aberrant vessels. The use of EUS in these settings has been limited mostly to patients with hemodynamically severe bleeding that was unresponsive to multiple prior interventions. Even within this challenging cohort, the reported clinical response rate is approximately 85%. However, despite the notable efficacy of EUS-guided angiotherapy in this broad patient cohort, the experience still is too limited, and the methodologies used are too divergent, to verify its efficacy, safety, and role in these settings. Nevertheless, the data highlight the potential utility and safety of EUS angiotherapy in managing clinically severe, refractory or recurrent hemorrhage in carefully selected patients.1,2
A 37-year-old male patient presented to the endocrinology clinic with confusion after 3 prior emergency department (ED) visits during which he was diagnosed with hypoglycemia. Initial evaluation included low blood glucose (42 mg/dL) with high beta-cell polypeptides and a negative sulfonylurea screen. Contrast-enhanced CT of the abdomen revealed a normal-appearing pancreas and no evidence of an insulinoma. Selective arterial calcium stimulation testing showed a profound spike of insulin in the gastroduodenal artery distribution, without appreciable change in the superior mesenteric artery or splenic artery distributions. This finding, consistent with an insulinoma of the head or neck of the pancreas, prompted referral for EUS examination.
EUS identified a hypervascular 13- by 9-mm insulinoma within the pancreatic neck anterior to the confluence of the splenic vein and superior mesenteric vein. Surgical consultation was obtained, and it was determined that, given the anatomic location of the tumor, enucleation was not feasible. Formal resection was considered, but the tumor location would have necessitated an extended distal pancreatectomy, leaving minimal pancreatic parenchyma and placing the patient at significant risk for pancreatic endocrine and exocrine insufficiency. Therefore, he was referred back to interventional gastroenterology for EUS-guided ethanol ablation.
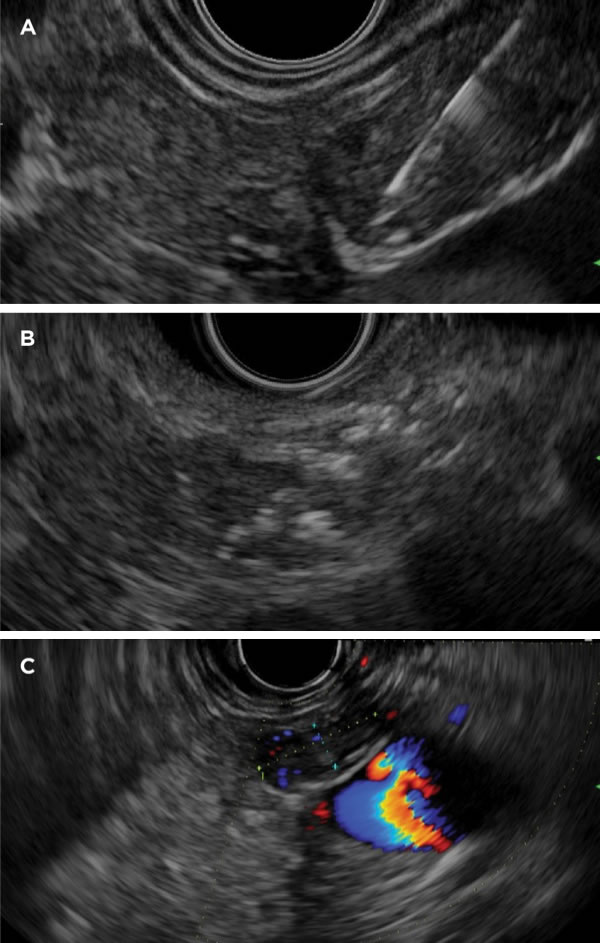
During EUS, we advanced a 22-gauge needle to the deepest aspect of the insulinoma (Figure 2A), injecting 98% (absolute) dehydrated ethanol slowly as the needle was withdrawn slowly. We injected several small aliquots, waiting approximately 30 seconds between each injection to allow the ethanol to permeate the tumor rather than extruding into extratumoral tissues. In total, we injected 0.42 mL of alcohol via 2 puncture sites to optimize the pattern and extent of spread. Subsequent Doppler examination showed a significant decrease in the vascular network, with hyperechoic changes of the tumor (Figure 2B). The patient's average morning glucose value increased to 80 mg/dL during the following month. Per our protocol, the patient underwent a second ablation 3 months later, during which 0.22 mL of 98% ethanol was injected. At that time, we noted the tumor to be smaller and to appear more hypoechoic (Figure 2C). Over the subsequent year, the patient remained asymptomatic and euglycemic. Although not yet in the clinical mainstream, EUS-guided ablation of pancreatic neuroendocrine tumors (NETs), as well as other malignancies, is a topic of ongoing research interest.3
A 49-year-old male patient with a history of metastatic cholangiocarcinoma, who was not a transplant candidate, presented with symptoms of right upper quadrant pain, fevers, and chills. He had undergone palliative uncovered transpapillary metal biliary stent placement for malignant biliary obstruction. Initial ED evaluation included abdominal CT, which demonstrated a hydropic gallbladder with a thickened wall and pericholecystic fluid. Given the patient's poor long-term prognosis and functional status, general surgery deferred cholecystectomy. Gastroenterology was consulted for cholecystitis treatment.
First, endoscopic retrograde cholangiopancreatography was attempted, with the goal of placing a transpapillary double-pigtail catheter into the gallbladder. Despite a forced occlusion cholangiogram, the cystic duct could not be identified, and blind guidewire passage failed to access the cystic duct, prohibiting transpapillary gallbladder stent placement. The patient was referred for EUS-guided stent placement. From a transduodenal view, we identified the dilated gallbladder, revealing an ideal window for transduodenal gallbladder drainage using an electrocautery-enhanced lumen-apposing metal stent (LAMS) (Axios, Boston Scientific). After Doppler ultrasound confirmed no intervening vascular structures, we located an ideal transduodenal puncture site that was less than 10 mm in thickness (Figure 3A). We advanced the LAMS-introducing catheter through the working channel of a therapeutic echoendoscope, and applied electrocautery while advancing the catheter through the duodenal wall and into the gallbladder body. We deployed the distal stent flange under EUS and fluoroscopic guidance within the gallbladder (Figure 3B). Slight retraction of the stent system allowed deployment of the proximal flange within the duodenal lumen, leading to immediate passage of a large volume of bile and pus and resulting in gallbladder decompression. The patient was discharged home one day later with complete resolution of his symptoms of cholecystitis and has remained without evidence of gallbladder disease at last follow-up 4 months after the procedure. The care of this patient demonstrates a safe alternative for gallbladder patients in whom standard techniques fail.4
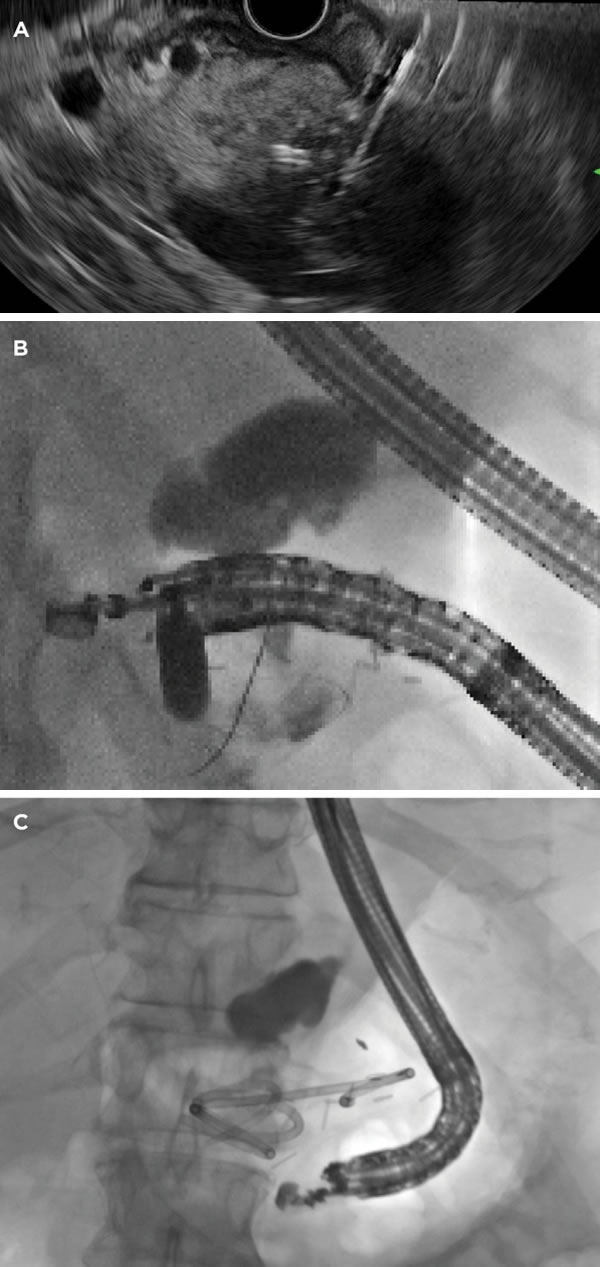
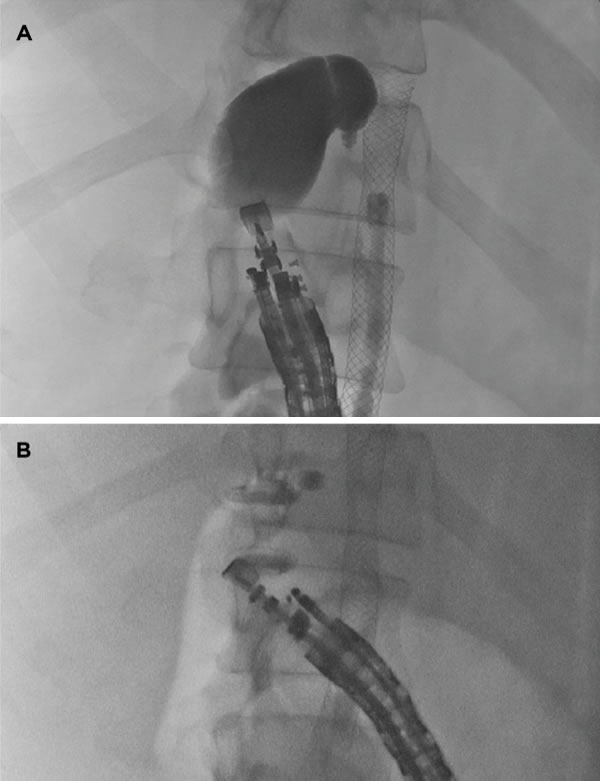
A 67-year-old male patient, who had been diagnosed with a well-differentiated NET measuring 3 cm in greatest dimension, underwent laparoscopic distal pancreatectomy and retroperitoneal lymphadenectomy. The surgery was complicated by postoperative fluid collection with fever and pain. CT showed a bilobed fluid collection in the operative bed, and there was no evidence that it was walled off. On postoperative day 5, the patient was referred for endoscopic drainage, and EUS was used to examine the fluid collection. The collection did not abut the gastric wall and had no apparent outer wall (Figure 4A), as had been seen on the prior CT scan. We entered the fluid collection via a transgastric puncture using a 19-gauge needle and aspirated pus from the collection. Full-strength ionic contrast injected into the cavity demonstrated a noncontained and diffuse pattern of distribution and further confirmed the absence of a wall. We fed a guidewire through the needle and coiled gently several times within this space (Figure 4B). Then, we dilated the cystogastrostomy tract using an 8-mm biliary dilation balloon catheter and placed two 7-Fr by 7-cm plastic double-pigtail stents with the distal pigtail within the fluid collection and proximal curled within the stomach (Figure 4C). The patient's pain and fever promptly resolved, and he was discharged from the hospital 2 days later. Two months after discharge, repeat CT showed resolution of the fluid collection, and the patient returned for esophagogastroduodenoscopy to remove both double-pigtail stents.
Although the concept of EUS-guided transluminal fluid collection drainage is not new, the care of this patient demonstrates the shifting indications and contraindications. Traditionally, acute postoperative fluid collections—defined as fluid collection occurring up to 30 days postoperatively—have been drained percutaneously. However, there is a growing body of evidence that patients with acute symptomatic fluid collections often may be able to avoid percutaneous drain placement and instead undergo EUS-guided internal transluminal drainage. The previously described requirement of a mature "walled-off" collection that closely apposes the gastric wall may, in fact, not be necessary. Given the absence of a containing wall and the lack of apposition with the gut lumen, consideration may be given to placement of a covered LAMS that may help seal the tract. However, as demonstrated in the care of this patient, use of double-pigtail plastic stents also may suffice.5
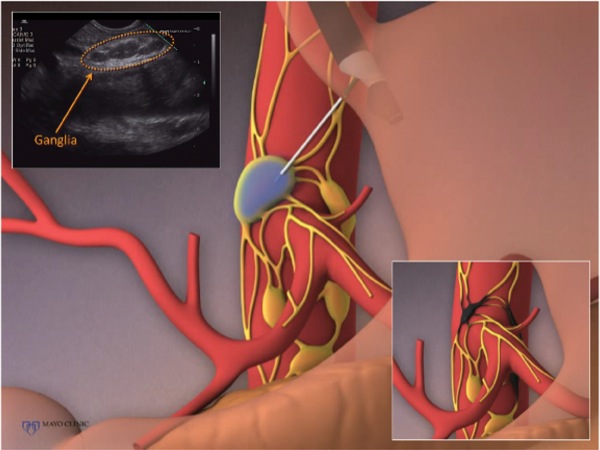
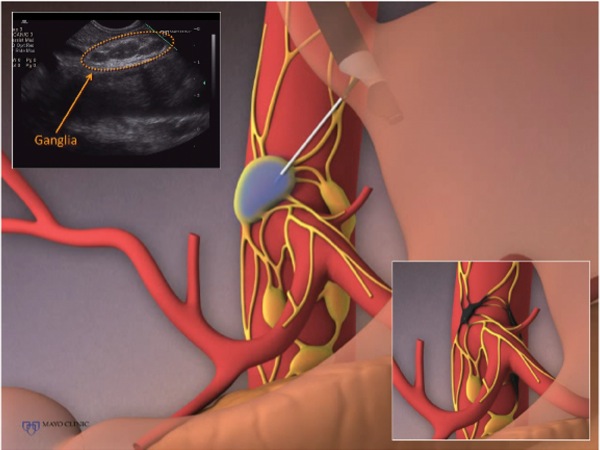
A 53-year-old female patient, recently diagnosed with unresectable adenocarcinoma of the tail of the pancreas, was referred by her oncologist for EUS-guided celiac plexus neurolysis to manage her substantial and escalating pain that was poorly responsive to high doses of narcotic analgesics. EUS examination of the celiac plexus demonstrated a chain of celiac ganglia (Figure 5). An injectate of 20 mL of absolute ethanol and 4 mL of bupivacaine was prepared for injection. Under EUS visualization using a 22-gauge FNA needle, we punctured 3 ganglia and injected 3 mL of injectate into each for a total of 9 mL for celiac ganglia neurolysis. We injected the remaining 15 mL of preparation in the standard fashion for bilateral celiac plexus neurolysis.
Celiac plexus neurolysis is a fairly s tandard therapeutic EUS technique, but the concept of direct ganglia injection is under investigation. Although celiac ganglia neurolysis theoretically offers greater pain relief, emerging data indicate a risk for shortened survival when compared with standard celiac plexus neurolysis.6
Although the indications and techniques for performing therapeutic EUS capture the attention of all prospective endosonographers, it is important to learn and remember the basics of diagnostic EUS that serve as an important foundation for developing therapeutic skills. A 57-year-old morbidly obese female patient with a history of liver transplant and no history of cardiac or respiratory disease presented for EUS to evaluate for a potential metastatic NET. Given her morbid obesity, the decision was made to intubate the patient for the procedure. Pulse oximetry recorded oxygen saturations of greater than 97% before intubation. Records indicate that at the start of the endoscopic procedure, the endotracheal tube (ETT) was positioned 22 cm from the incisors and anesthesia staff confirmed appropriate placement on auscultation. During the procedure, the patient experienced unexplained desaturation despite pulmonary recruitment maneuvers.
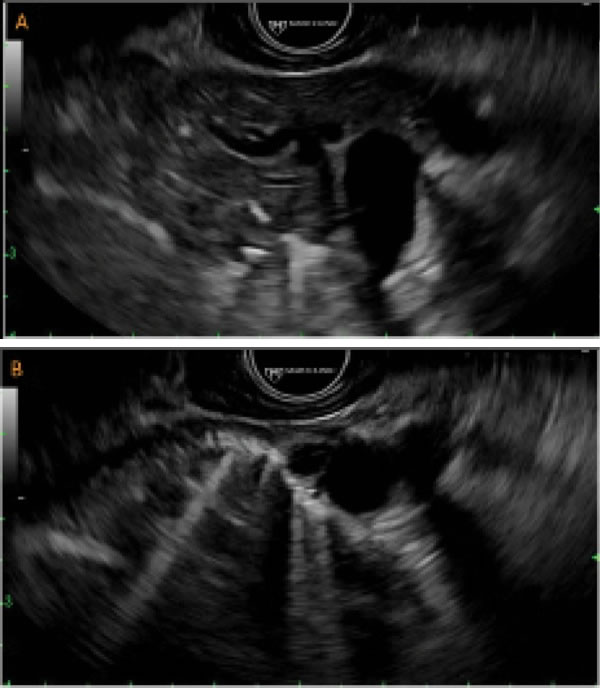
During EUS, it was noted that the lung parenchyma was easily visualized (Figure 6A), which is not normal. When the lungs are filled with air, there is a large reflection coefficient, which largely results from the difference in acoustic impedance between soft tissues and air. As a result, sufficient echo amplitudes cannot be recovered from tissues beyond this interface. The ability to image the lung parenchyma was a clue that the airway was collapsed and contained minimal air due to atelectasis secondary to malposition of the ETT. After concern was raised about right mainstem bronchial intubation, the anesthetist evaluated the depth of the ETT placement and retracted to 20 cm; this resulted in rapid reinflation of the left lung. This process was monitored during EUS with detection of air inflow and loss of parenchymal visualization (Figure 6B). The procedure was completed without further respiratory compromise, and the patient was successfully extubated at completion of examination and discharged home from the endoscopy unit without postprocedural complications.
Malposition of an ETT may result in inadvertent right mainstem bronchial intubation, which can cause serious complications including hypoxemia, contralateral atelectasis, and ipsilateral hyperinflation and barotrauma. Bronchial intubations may occur in up to 5% to 8% of intubations, with higher rates in women and during emergency intubations. Standard placement depth of an ETT measured at the patient's teeth is 22 to 23 cm in men and 20 to 21 cm in women. For endoscopists performing procedures with general anesthesia, it is an important consideration in the differential diagnosis of intraprocedural hypoxia.7
Therapeutic EUS is an exciting and evolving field within gastroenterology. Improvements in ultrasound imaging technology and diversity of endoscopic tools and devices have led to rapid developments in the field. Some of the capabilities of EUS-guided therapeutic procedures have been highlighted in the cases presented, including angiotherapy for a bleeding splenic aneurysm, ethanol ablation and resolution of a symptomatic insulinoma, placement of a LAMS to palliate cholecystitis, drainage of an acute postsurgical fluid collection, and interventional pain management via celiac ganglia neurolysis. In our final case, we also reemphasize the need to develop and maintain strong fundamental diagnostic and problem-solving skills to enhance the efficacy and safety of all EUS procedures.