For many years, endoscopic ultrasound (EUS) was used solely as a diagnostic test to enhance tumor staging and characterize mucosal and submucosal lesions. More recently, the applications of EUS have been expanded to an alternative, and increasingly primary, role in the guidance of therapeutic interventions across a broad spectrum of gastrointestinal and nongastrointestinal disorders. Herein, we present selected patients in whom EUS provided a novel approach to patient care.

A 53-year-old woman with a history of morbid obesity presented with severe abdominal pain and was admitted to the medical ICU with necrotizing pancreatitis and concomitant biliary obstruction. The patient was stabilized medically and underwent successful endoscopic retrograde cholangiopancreatography (ERCP) for sphincterotomy, with clearance of a small common bile duct stone. She was stabilized and discharged home 2 weeks after her admission, but she returned 3 weeks later with failure to thrive characterized by the inability to tolerate solid foods, severe nausea, and ongoing mid-abdominal pain. Repeat cross-sectional imaging with magnetic resonance cholangiopancreatography revealed a 12-cm area of peripancreatic walled-off necrosis, with more than 60% internal solid necrosis based on MRI findings.
Referral for endoscopic necrosectomy led to EUS examination confirming the presence of a large necrotic collection in the body/tail junction of the pancreas, posterior and inferior to the body of the stomach (Figure 1A). The collection was accessed using a new 20-mm electrocautery-enhanced lumen-apposing metal stent deployment catheter (Boston Scientific), and the stent was deployed across the cystogastrostomy tract. The stent was balloon dilated to the full 20-mm aperture using a through-the-scope balloon dilation catheter. A large amount of fluid and debris was seen passing through the stent into the stomach. Less than 1 hour of debridement was undertaken to remove some of the necrotic material (Figure 1B). In its final appearance, the cyst had clean pink walls without significant residual necrosis. Finally, a soft double pigtail catheter was placed to maintain patency of the lumen-apposing metal stent (Figure 1C).
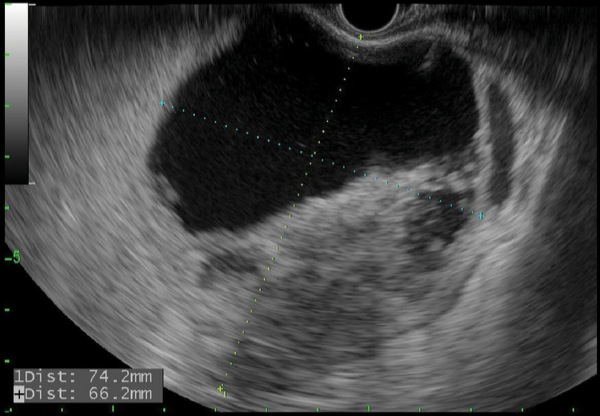
Figure 1A.
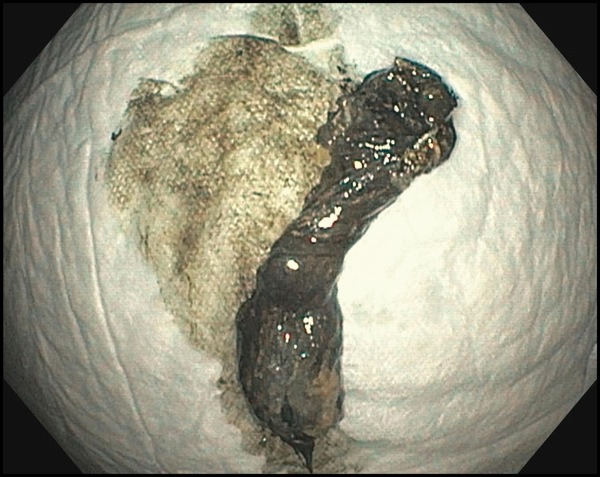
Figure 1B.
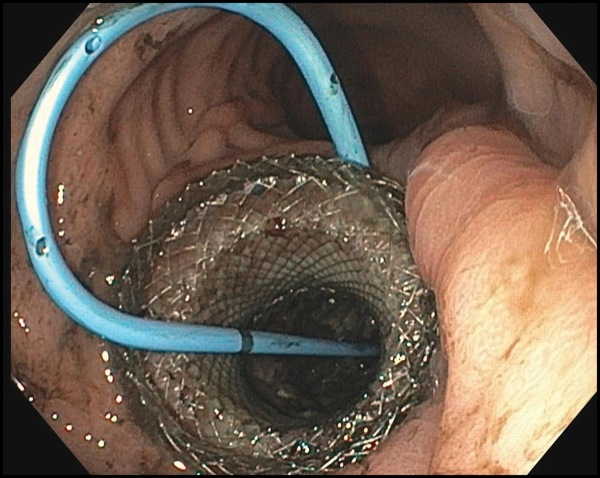
Figure 1C.
Two weeks later, repeat CT showed the patient’s collection had decreased in size to just less than 5 cm, and she reported an increased ability to tolerate an oral solid diet. Repeat imaging an additional 2 weeks later confirmed resolution of the collection to less than 2 cm. Endoscopy to remove the stent was performed, and the patient underwent laparoscopic cholecystectomy. She remained asymptomatic 9 months after her procedure.
Two weeks later, repeat CT showed the patient’s collection had decreased in size to just less than 5 cm, and she reported an increased ability to tolerate an oral solid diet. Repeat imaging an Lumen-apposing metal stents have revolutionized therapeutic EUS. As demonstrated in this case, the majority of symptomatic walled-off necroses may be managed successfully and with minimal morbidity compared with that observed using previously preferred surgical techniques.1 A US study led by Mayo Clinic is underway to support FDA approval of the device, including larger 20-mm versions of the stents, for this indication.
A 67-year-old woman with recurrent rectal cancer underwent resection of a pelvic mass, left colectomy, intraoperative radiation therapy, left nephrectomy, and aortobiiliac reconstruction with an aortoiliac bypass allograft. Shortly after this surgery, the patient was noted to be febrile with new pelvic fullness. Cross-sectional imaging revealed a developing abscess in the tumor resection bed, adjacent to the rectal stump. Intravenous antibiotics were given to prevent infection of the nearby aortoiliac bypass graft. Given her sepsis and recent radical surgeries, there was concern about reoperating, and the location of the collection was believed to be difficult for percutaneous drainage. Because of this, the patient was referred for EUS-guided transrectal internal drainage.
EUS revealed an 8-cm perirectal, poorly defined (no appreciable wall) fluid collection with internal necrosis (Figure 2A). With a 19-gauge needle, the collection was punctured and a 0.035-inch wire coiled several times within the collection. The tract was dilated to 6 mm with a biliary balloon dilation catheter, and then 2 soft double pigtail stents (10 Fr by 5 cm) were placed across the stoma (Figure 2B-C). Serial imaging revealed improvement in the collection at 4 weeks, and near-complete resolution at 3 months. The stents were removed, and the patient remained asymptomatic and afebrile without antibiotics.
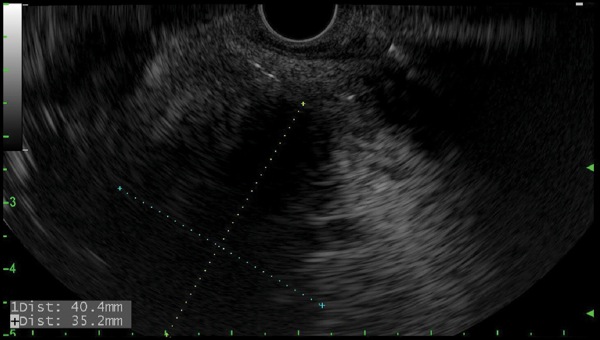
Figure 2A.
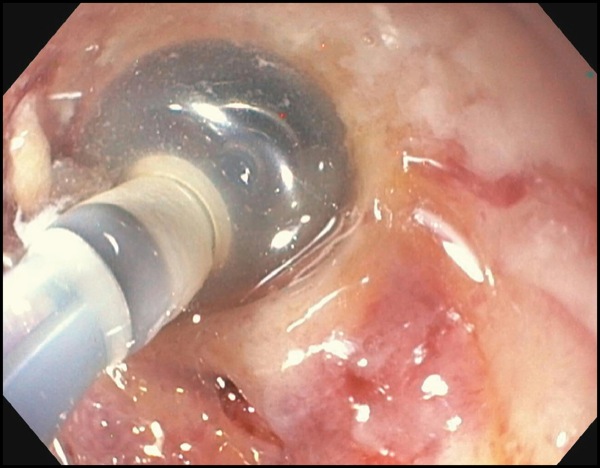
Figure 2B.
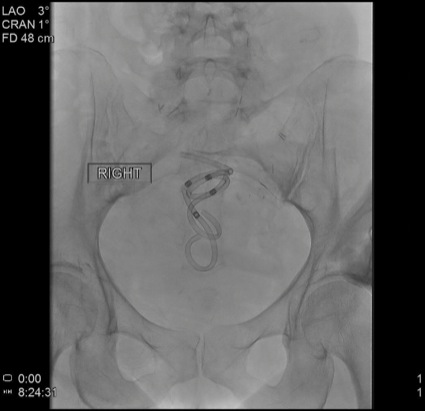
Figure 2C.
Similar to drainage of acute postsurgical collections,2 perirectal collections, particularly when below the peritoneal reflection, may be a common, future site of therapeutic EUS-guided intervention.
A 48-year-old woman who had undergone Roux-en-Y gastric bypass for weight loss presented with abdominal pain and jaundice. Cross-sectional imaging was suggestive of a mass in the head of the pancreas. Upper endoscopy revealed a long gastric pouch, extending 8 cm beyond the gastroesophageal junction; EUS was attempted for tissue diagnosis.
Fine needle aspiration (FNA) was performed, and in-room cytology confirmed pancreatic adenocarcinoma that appeared to involve the descending duodenum and peripancreatic fat on EUS imaging. Long-limb ERCP using a single-balloon enteroscope was able to access the area of the papilla, but cannulation could not be achieved.
Given this, EUS-guided biliary rendezvous was performed. From the gastric pouch, a dilated intrahepatic duct in the left lobe of the liver was visualized. This was punctured using a 19-gauge FNA needle, and contrast was injected to complete a cholangiogram (Figure 3A). The needle was flushed with sterile saline, and a 0.025-inch angled hydrophilic wire was passed through the needle and guided across the distal biliary stricture. The wire was then advanced as far as possible into the Roux limb to the jejunojejunostomy. The EUS scope was then removed over this wire, and alongside the wire an adult colonoscope was passed to the level of the jejunojejunostomy, where the guidewire was visualized, grasped with forceps, and internalized through the colonoscope. The wire was then held tightly from both the transoral end and trans-colonoscope end, and the colonoscope was advanced over this wire serving as a rail to the level of the papilla in the second portion of the duodenum (Figure 3B). Over this guidewire, a small needle-knife sphincterotomy was performed, followed by balloon sphincteroplasty. Finally, an uncovered metal biliary stent was placed for palliation of the patient’s malignant biliary obstruction (Figure 3C).
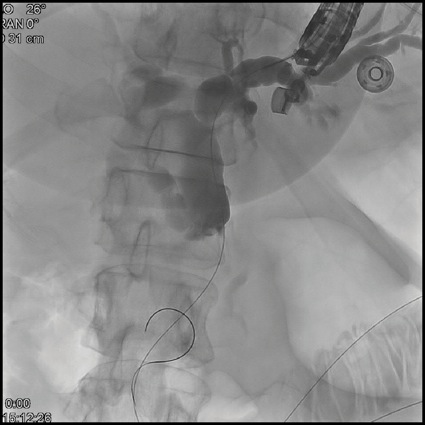
Figure 3A.
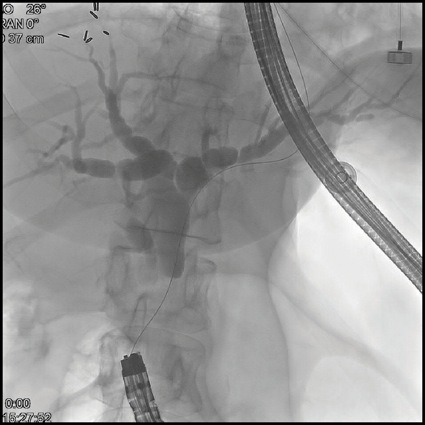
Figure 3B.
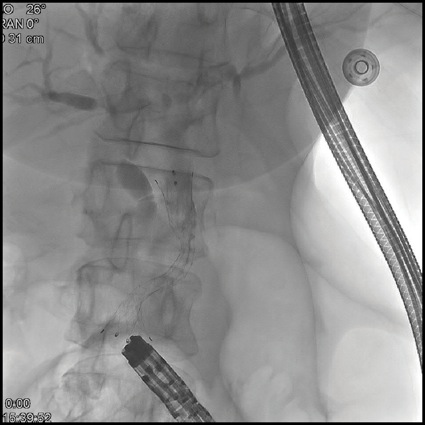
Figure 3C.
Multiple means of biliary access have been described for Roux anatomy ERCP including, but not limited to, interventional radiology rendezvous, surgical duodenoscopic access to the remnant stomach, percutaneous endoscopic gastrostomy tract access to the remnant stomach, creation of a gastrogastric fistula using an EUS-guided lumen-apposing metal stent, and, more recently, interventional radiology–assisted percutaneous transhepatic transpapillary wire placement.3 This case highlights one of several purely endoscopic, and specifically EUS-guided, approaches to very challenging long-limb anatomy ERCP.
A 47-year-old man with a history of rectal adenocarcinoma and surgical resection with repeat colo-colonic anastomosis, radiation therapy, and chemotherapy presented with nausea, vomiting, dehydration, and obstructive jaundice. Abdominal CT demonstrated a large pancreatic head mass and duodenal obstruction (Figure 4A). ERP was attempted but failed because of duodenal tumor ingrowth, an inability to visualize the papilla, and duodenal obstruction (Figure 4B). The patient was referred for EUS-guided palliation of his obstructive jaundice.
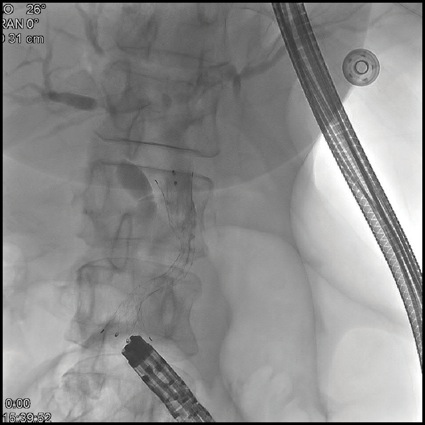
Figure 4A.
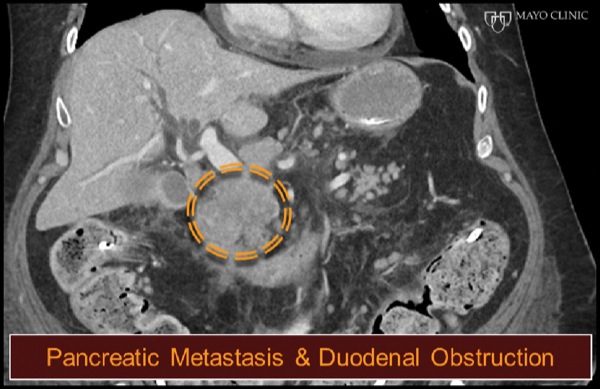
Figure 4B.
At EUS, the jejunum was visualized after contrast insufflation, thereby providing a target for placement of a lumen-apposing metal stent and creation of a gastrojejunostomy (Figure 4C). The region of extrahepatic bile duct that could be accessed from the bulb measured only 5 mm and was believed to be a suboptimal target for placement of the lumen-apposing metal stent (Figure 4D). The gallbladder was distended and communicated with the extrahepatic bile duct via a patent cystic duct, thereby serving as an ideal target for biliary decompression (Figure 4E-F). The gastrojejunostomy and cholecystoduodenostomy successfully restored the patient’s ability to tolerate oral intake and resolved his cholestasis (Figure 4G).
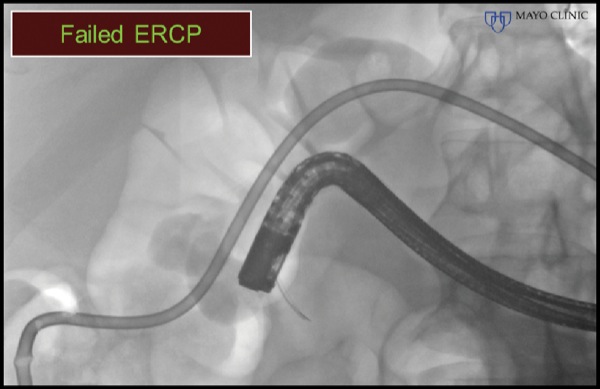
Figure 4C.
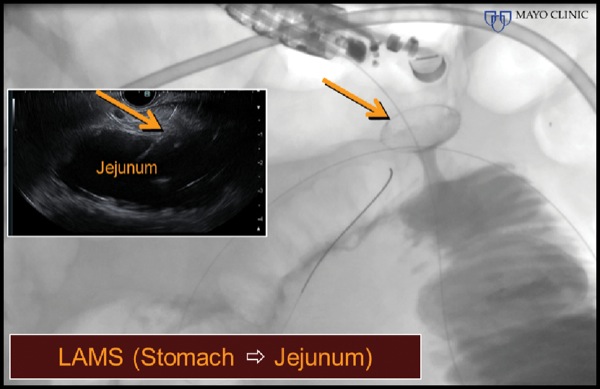
Figure 4D.
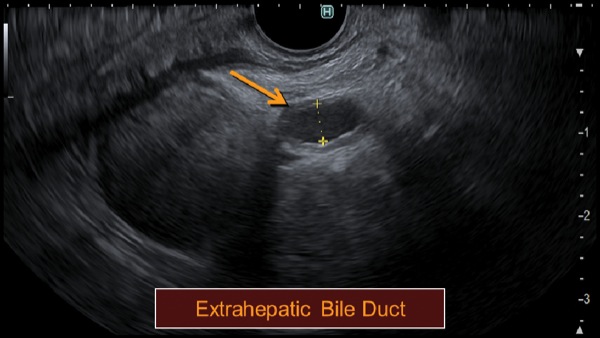
Figure 4E.
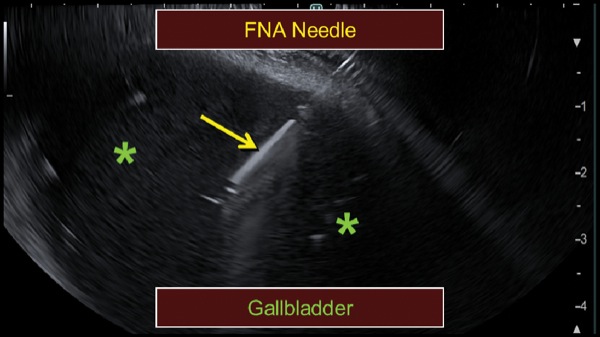
Figure 4F.
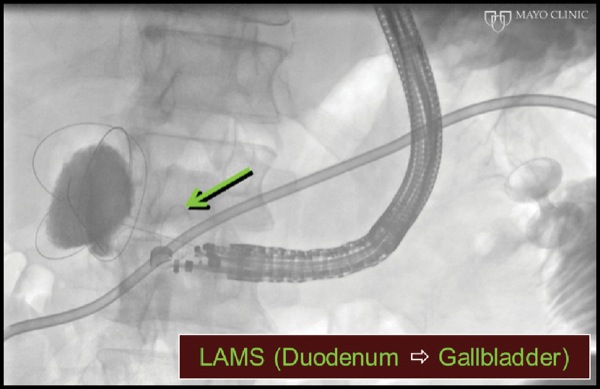
Figure 4G.
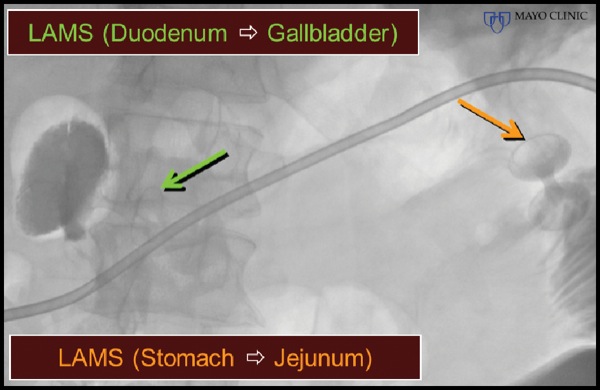
Figure 4G.
The care of this patient highlights the therapeutic capability of EUS in patients with malignant biliary and duodenal obstruction, allowing a single-session biliary decompression and duodenal bypass.
ERCP, endoscopic retrograde cholangiopancreatography; FNA, fine needle aspiration; LAMS, lumen-apposing metal stent
An 85-year-old woman with multiple comorbid illnesses presented with abdominal CT findings of a markedly dilated main pancreatic duct and numerous large side branches throughout the pancreas as a manifestation of cytologically confirmed malignant transformation of intraductal papillary mucinous neoplasm of the pancreas (Figure 5A-B). The patient was referred for EUS-guided biliary drainage after a failed ERCP. EUS also demonstrated the main pancreatic duct dilatation and numerous enlarged side branches, along with a markedly dilated extrahepatic bile duct (Figure 5C).
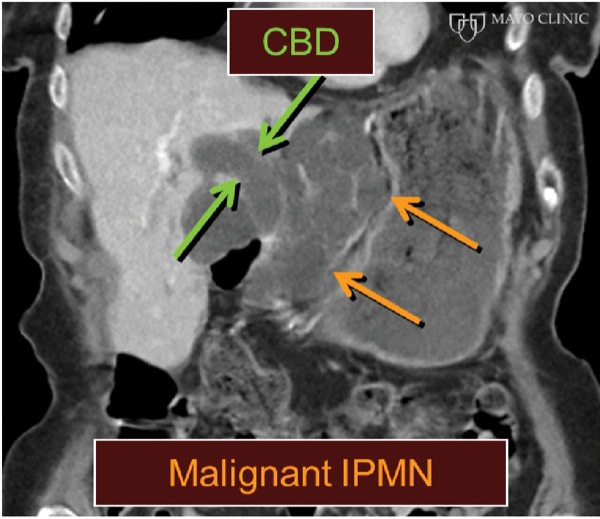
Figure 5A.
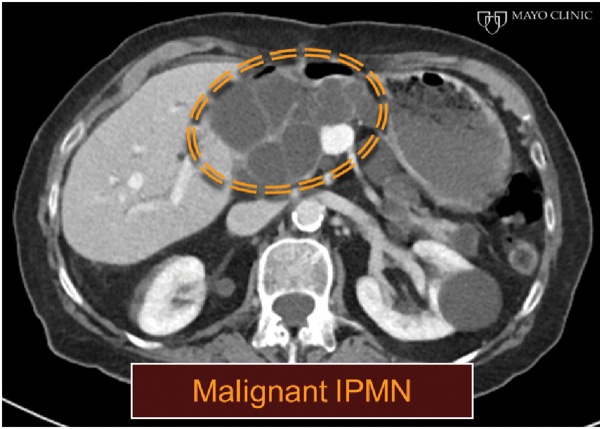
Figure 5B.
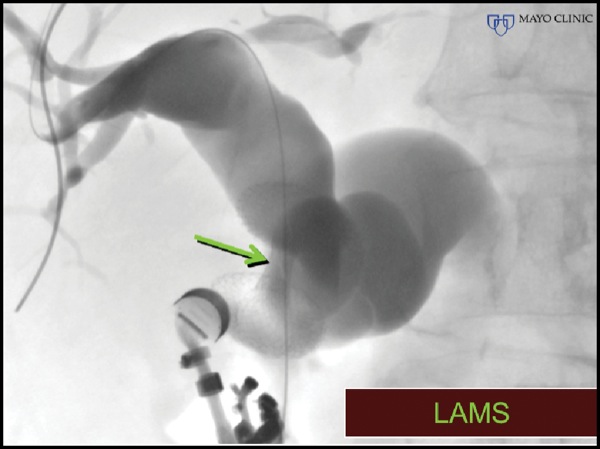
Figure 5C.
An Access Needle (Beacon, Medtronic) was inserted into the extrahepatic bile duct and torqued to advance the guidewire retrograde into the intrahepatic bile ducts (Figure 5D-E). This maneuver was followed by placement of a lumen-apposing metal stent.
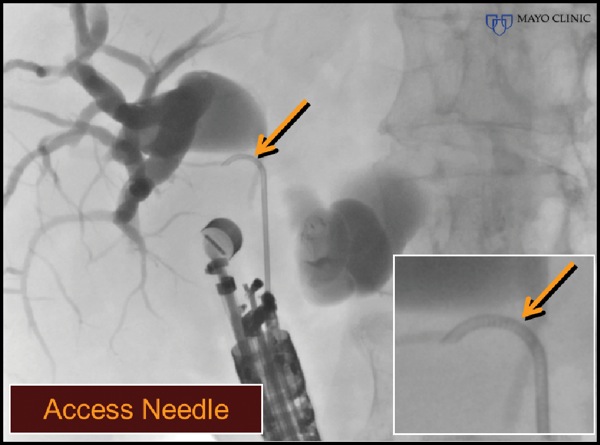
Figure 5D.
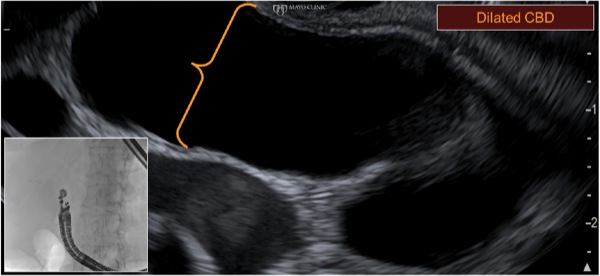
Figure 5E.
The care of this patient highlights the potential utility of new needles that provide some “steerability,” which has been available to our interventional radiology colleagues but not seen within the toolbox of therapeutic EUS. Their ability to accurately torque the needle tip trajectory enhances guidewire placement and, if needed, subsequent stent insertion. In this patient, given the trajectory of the echoendoscope, a standard needle also would have provided free access to the proximal ductal anatomy. However, transduodenal access from the bulb often complicates passage of the guidewire in the opposite trajectory into the distal bile duct and into a transpapillary position. These needles are likely to facilitate intrahepatic and pancreatic transluminal and rendezvous maneuvers as experience with their use becomes more widespread.
CBD, common bile duct; IPMN, intraductal papillary mucinous neoplasm; LAMS, lumen-apposing metal stent
A 74-year-old woman with cholangiocarcinoma underwent combined ERCP (for biliary drainage) and EUS (for staging purposes). At EUS, a thrombus was identified within the portal vein, hepatic veins, and inferior vena cava, extending into the right atrium (Figure 6A-B). After discussion with hepatology and transplant colleagues, we decided to proceed with EUS FNA to differentiate a bland versus malignant thrombus, given that the findings would substantially affect the patient’s clinical care. EUS FNA of the portal vein thrombus was positive for malignancy, and the patient was managed in a conservative manner (Figure 6C).
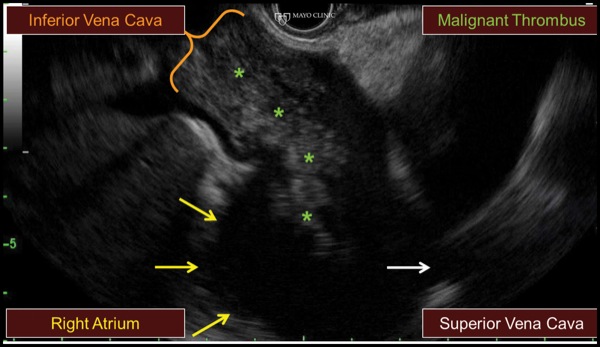
Figure 6A.
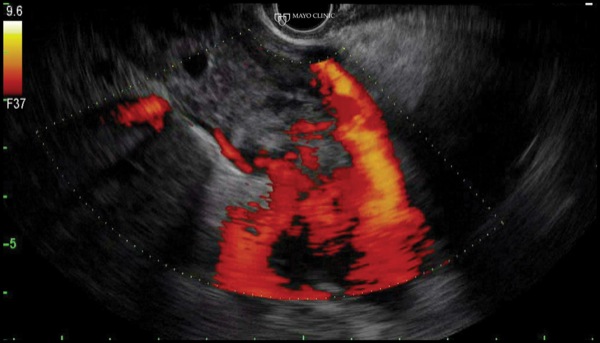
Figure 6B.
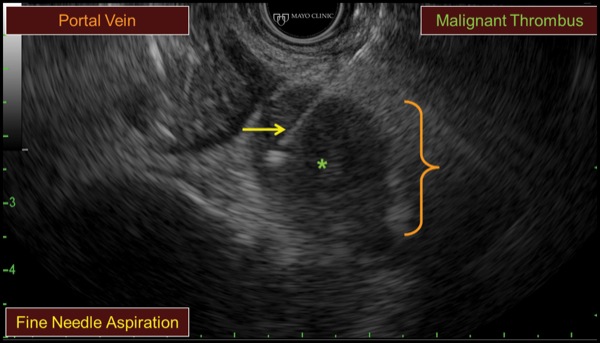
Figure 6Cs.
Although malignant thrombi often can be identified easily, they may be missed due to both a lack of consideration and because a thrombus may be obscured within the liver due to the similar echodensity and echopattern between the thrombus and hepatic parenchyma. Careful inspection is needed to see occult thrombi.
Given that EUS imaging has uncertain accuracy in distinguishing malignant from bland thrombi, we advocated for use of EUS FNA, which can be performed safely. We suggest doing so only when the findings will alter patient care and after discussion with the referring team.
We suggest a few technical considerations that may decrease the risk and enhance the diagnostic accuracy of vascular FNA, including:
The spectrum of therapeutic EUS capabilities is rapidly expanding. Enhanced ultrasound imaging technology and a growing availability of dedicated endoscopic tools and devices are fueling this growth. Some of the capabilities of EUS-guided therapeutics have been highlighted in the cases presented here, including new larger lumen-apposing metal stents for walled-off necrosis, transrectal collection drainage, biliary rendezvous in surgically altered anatomy, placement of lumen-apposing metal stents to palliate biliary obstruction and luminal obstruction, and use of a new steerable FNA access needle.
Finally, we reemphasize the importance of the diagnostic capacities of EUS that should be just as actively sought by endosonographers as the exciting and growing therapeutic capabilities. Possession of either skill set alone restricts the procedural capabilities and limits the potential impact of this tool on patient care.